Q:
Which of the following reactions are redox reactions?
Answer & Explanation
Answer: D) All of the above
Explanation: A redox reaction is a type of reaction which involves transfer of electrons among two species. This reaction is also called oxidation-reduction reaction.
In oxidation process, there is an increase in oxidation state of an atom, ion or molecule by losing the electrons.
In reduction process, there is a decrease in oxidation state of an atom, ion or molecule by accepting the electrons.
1)
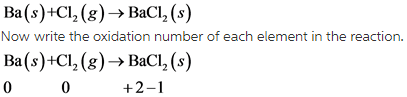
In this reaction, Ba undergoes oxidation and Cl undergoes reduction.
This reaction is redox reaction.
In this reaction, oxidation number of Ba increases from 0 to +2 . So, Ba undergoes oxidation. The oxidation number of Cl decreases from 0 to -1. So, Cl undergoes reduction.
2)
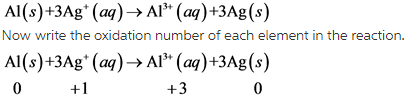
From this above reaction, it is clear that Aluminum undergoes oxidation and silver undergoes reduction.
Due to this, this reaction is redox reaction.
In this reaction, oxidation number of Aluminum increases from 0 to +1. So, aluminum undergoes oxidation. The oxidation number of silver decreases from +1 to 0. So, silver undergoes reduction.
3)
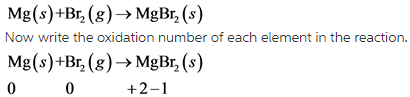
In this reaction, Mg undergoes oxidation and Br undergoes reduction.
This reaction is redox reaction.
The reactions 1, 3 and 4 are redox reactions.
In this reaction, oxidation number of Mg increases from 0 to +2 . So, Mg undergoes oxidation. The oxidation number of Br decreases from 0 to -1 . So, Br undergoes reduction.
Hence all the above reactions are redox reactions.
View Answer
Report Error
Discuss