| A) SO2 | B) CH2Cl2 |
| C) PCl3 | D) All the above |
Explanation:
1) SO2 2) CH2Cl2 3) PCl3
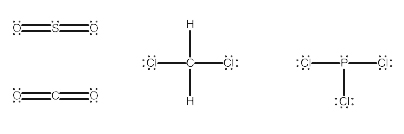
How to check the given molecules are polar or non polar :
If the electronegativity of the two bonded atoms are different then the bond is polar. Although, some molecules contain polar bonds, the molecule may be non-polar. If the polarity of all the bonds present in the molecule are cancel each other then molecule is non-polar.
In the given options,
SO2 is polar because the dipole moments on the O do not cancel.
PCl3 is polar because the P-Cl bonds do not cancel out.
CH2Cl2 polar because the C-Cl bonds do not cancel.
Hence, in brief, Polar molecules are molecules with net dipole due to the presence of partial positive and partial negative charge.
For example, water is a polar molecule that has partial positive and partial negative charge on it. CO2 is a non polar compound.
Therefore, all the given options SO2, CH2Cl2 and PCl3 are Polar molecules.

 Aptitude and Reasoning
Aptitude and Reasoning General Knowledge
General Knowledge Puzzles
Puzzles Interviews
Interviews Technical
Technical Certifications
Certifications Exams
Exams Job
Roles
Job
Roles True or False
True or False Exams
Exams